News
WBVR awarded with 25 million CEPI grant for Rift Valley fever vaccine research
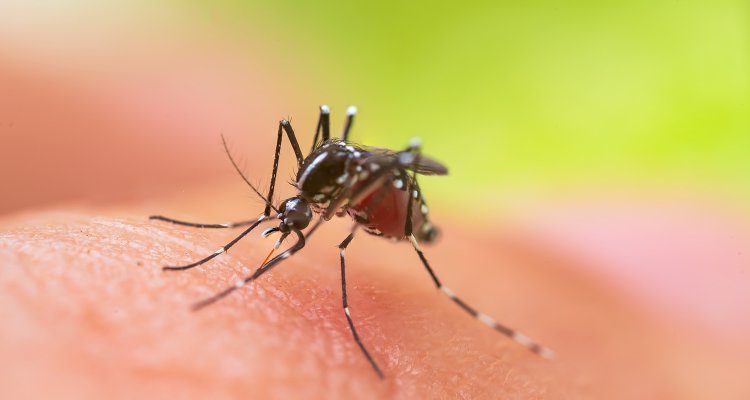
The Coalition for Epidemic Preparedness Innovations (CEPI) is expanding its partnership with Wageningen Bioveterinary Research (WBVR, part of Wageningen University & Research) to advance its vaccine candidate against the deadly mosquito borne Rift Valley fever virus (RVFV). WBVR will lead a consortium to perform clinical trials in East Africa in people most at risk of infection.
CEPI will invest up to $25.9 million, with support from the EU, in trials to assess safety and immunogenicity of a live-attenuated vaccine known as hRVFV-4s against Rift Valley Fever (RVF) in people most at risk of infection. WBVR will use the support from CEPI to lead a consortium that will conduct a Phase I/IIa clinical trial in East Africa. “These trials will help to accelerate the urgent need for a protective vaccine. Besides offering protection in areas were the virus is already active, a RVF vaccine will contribute to preparedness for potential virus emergence in for instance Europe”, says Paul Wichgers Schreur, researcher at WBVR, developer of the hRVFV-4s vaccine and leader of the consortium.
Deadly virus
RVF is a potentially deadly virus which can spread to people either through contact with blood, body fluids or tissues of infected livestock or by mosquito bites. The disease can cause severe disease with symptoms such as encephalitis and haemorrhaging and kills around 1 percent of all those it infects. RVF is also profoundly destructive for the livelihoods of those in rural areas where outbreaks occur and often results in large-scale losses of livestock. Because of its impact on both people and animals, RVF is a prime candidate for a ‘One Health’ approach to disease control. No safe and effective human vaccines or treatments have yet been approved for use against RVF, so their development is considered a top priority by both the Africa CDC and the World Health Organization’s R&D Blueprint team.
Earlier trials
A Phase I clinical trial in Belgium, supported by a previous CEPI/EU grant, is currently being finalized using the same vaccine. “In this first in human clinical trial promising results were seen that formed the basis for the continuation of our partnership with CEPI,” says Wichgers Schreur.
Partnership
“We urgently need a vaccine to strengthen our defences against the emerging disease Rift Valley Fever and thus protect the vulnerable populations who are exposed to it. Through CEPI’s expanded partnership with Wageningen Bioveterinary Research, supported by EU funding, we will generate crucial data needed to accelerate the development of this much-needed vaccine, helping to bring a protective solution closer to the growing number of people who may be affected by this potentially deadly disease,” states dr. Richard Hatchett, CEO of CEPI.
Essential research
“An effective vaccine against Rift Valley Fever would go a long way to prevent more frequent and deadly outbreaks, with all the serious public health and socioeconomic consequences that we see today. I am pleased to see that these essential research projects can now take off with the steadfast support of the European Union and Horizon Europe, through our great collaboration with CEPI”, according to Marc Lemaître, Director-General for Research and Innovation, European Commission. “I am confident that the projects starting today will bring us even closer to an effective vaccine against Rift Valley Fever.”